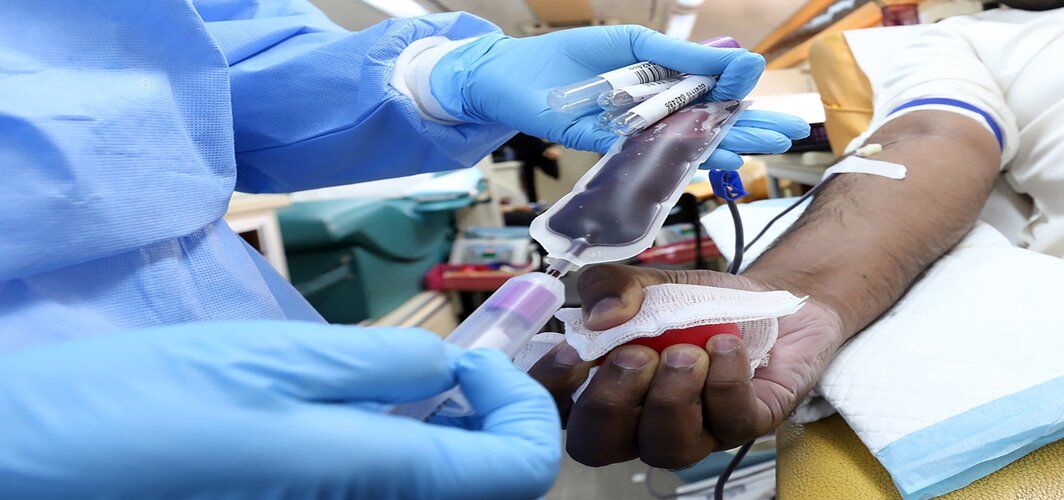
After the announcement by the U.S Food and Drug Administration (FDA) in late August about the emergency use of convalescent plasma therapy to treat severely ill COVID-19 patients, several hospitals worldwide have started administering the same. A few states in India had backed the therapy several weeks earlier, and doctors could prescribe it to certain patients. The therapy is being used as a treatment option in patients with moderate to severe COVID-19 complications. The convalescent plasma therapy received a lot of attention during the Ebola, Junin virus, SARS, MERS, and H1N1 pandemics. Despite limited evidence of the plasma treatment therapy for the Coronavirus, many healthcare experts believe that it can help prevent the progression of COVID-19 symptoms. As scientists and doctors continue to learn and gather more evidence to prove the effectiveness of the convalescent plasma therapy in treating COVID-19 infected patients, here's what we already know about this therapy.
What is convalescent plasma therapy and how does it work?
After getting infected with the Coronavirus, an individual’s immune system starts generating antibodies to fight off the infection. These antibodies are basically proteins that are found in the blood plasma. Convalescent plasma derived from the blood of a person who has recovered from COVID-19 is transferred intravenously to the newly infected patient who doesn’t possess the antibodies or immunity required to fight off the Coronavirus. This plasma therapy or convalescent plasma therapy induces immunity in the infected patient until the patient's immune system starts generating its own antibodies in an adequate amount, to recover from COVID-19.
The donor can donate their plasma 24 times in a year and up to twice a week with a gap of 48 hours or more. However, there are some rules to donate plasma for convalescent therapy. The donor who is supposed to donate plasma must have:
- Survived/recovered from the COVID-19 infection and should be free of any disease or symptoms for at least 28 days.
- Tested negative for the Coronavirus infection at least 14 days before their plasma can be used for the therapy.
Additional eligibility criteria for donating convalescent plasma
Besides the above criteria, donors who wish to donate their plasma should also satisfy the following conditions:
- Should be more than 18 years of age or older.
- Should have a respiratory rate of 24/min and oxygen saturation level of 93% in room air
- Should not be a pregnant or a breastfeeding woman
- Should not have any hypersensitivity towards blood products
- Should not be critically ill
- Should weigh 55kgs or above
- Should not have IgA deficiency or immunoglobulin allergy.
Uncertainties and limitations in the use of convalescent plasma therapy
Convalescent plasma therapy has been effective in treating several infections in conjunction with other drugs and preventive measures. However, there are relatively few studies showing its efficacy and safety levels in delivering promising results. There are still some ongoing debates and issues regarding its implementation on a large-scale. Limited high-quality studies and randomized clinical trials, selection of donors who possess high neutralizing antibody titers, and fatality rates are some of the significant factors that impact the efficacy of convalescent plasma therapy.
While convalescent plasma treatment has been used for over 100 years now, many experts are still sceptical about its large scale administration due to potential health risks that include allergic reactions and transfusion-associated acute lung injury, also known as TRALI, difficult breathing, and transmission of infections such as hepatitis B and HIV.
What does the data tell us?
As per a recent program conducted by the US FDA and sponsored by the National Institutes of Health, convalescent plasma (CCP) therapy was given to 35,000 COVID-19 patients. Although many patients improved clinically, this therapy's specific role could not be determined as all the infected patients received at least one supplementary treatment, including antivirals, antibiotics, antifungals, and corticosteroids. The mortality rate was slightly lower, around 8.7%, in the patients who received convalescent plasma within three days of Coronavirus diagnosis. And in patients who received convalescent plasma after four days of diagnosis, the mortality rate was recorded at 11.9%. Despite such stats on the convalescent plasma therapy's efficacy levels, the scientific community needs more time and research to broaden their understanding and determine how impactful the plasma treatment might be, to treat or manage Coronavirus symptoms.
Which states in India have approved convalescent plasma therapy for COVID-19 patients?
After the approval from the Union Health Ministry and Drug Controller General of India (DCGI) on using convalescent plasma therapy on COVID-19 patients, many states in India have directed the blood banks to collect and store convalescent plasma from the Coronavirus-recovered patients. As a crucial step to improve and simplify access to blood plasma for treating Coronavirus patients, Delhi set up the country's first blood plasma bank in July 2020. Delhi was one of the first states in the country to start convalescent plasma therapy in April. Many other states such as Maharashtra, Punjab, Rajasthan, Karnataka, Goa, and Odisha followed the lead and implemented convalescent plasma therapy to treat COVID-19 patients.
Conclusion
Till date, there are no robust pieces of evidence to suggest that plasma therapy can be used as the definitive and sole treatment for COVID-19. Though health apex bodies like the U.S. FDA and Indian Council of Medical Research (ICMR) have approved the use of plasma from COVID-19 survivors for investigative trial purposes, experts stress the importance of carrying out well-conducted research trials before administering plasma therapy for routine use in COVID-19 patients. As of now, only a few data sets gathered from small randomized clinical trials support that convalescent plasma may lessen the severity of COVID-19. More research is needed for drawing a conclusion on the long-term benefits of convalescent plasma therapy in the context of COVID-19.
If you have any questions, you can:
Talk to a COVID-19 Expert