Guide to Car T Cell Therapy
Explore CAR T-cell therapy, a revolutionary cancer treatment, including how it works, its benefits, potential side effects, and patient considerations.

Written by Dr. Md Yusuf Shareef
Reviewed by Dr. Rohinipriyanka Pondugula MBBS
Last updated on 13th Jan, 2026
.webp?tr=q-80,f-webp,w-350,dpr-2,c-at_max 700w)
Introduction
Cancer treatment has witnessed a paradigm shift with the advent of immunotherapy, and at the forefront of this revolution is CAR T-cell therapy. This groundbreaking approach doesn't involve traditional methods like chemotherapy drugs or radiation beams. Instead, it harnesses the power of your own immune system to fight cancer. It’s a highly personalised and sophisticated treatment that has shown remarkable success in patients with certain blood cancers who have not responded to other therapies. This guide will demystify CAR T-cell therapy, explaining how it works, who it can help, what the process entails, and the potential benefits and risks. Whether you are a patient, a caregiver, or simply curious about medical advancements, this article will provide a comprehensive overview of this powerful therapy.
What is CAR T-Cell Therapy? A Basic Breakdown
CAR T-cell therapy, short for Chimeric Antigen Receptor T-cell therapy, is a type of immunotherapy. In simple terms, it’s a process where a patient’s own immune cells are genetically modified to better recognise and destroy cancer cells.
Think of your T-cells as the soldiers of your immune system. Normally, they patrol your body looking for invaders like viruses and bacteria. However, cancer cells are clever; they can disguise themselves to evade detection by these soldiers. CAR T-cell therapy works by taking a patient's T-cells from their blood, genetically engineering them in a laboratory to produce special receptors called chimeric antigen receptors (CARs) on their surface. These CARs act like highly precise GPS systems, allowing the T-cells to recognise and latch onto specific proteins (antigens) found on cancer cells. Once these "supercharged" CAR T-cells are infused back into the patient, they multiply and launch a targeted attack on the cancer.
How is it Different from Chemotherapy or Radiation?
Traditional treatments like chemotherapy and radiation are like broad-spectrum attacks. They work by killing rapidly dividing cells, which includes cancer cells but also harms healthy cells like those in hair follicles, the mouth, and the digestive system, leading to well-known side effects.
- Targeted Action: CAR T-cell therapy is far more precise. The engineered cells specifically hunt down cancer cells bearing the target antigen, largely sparing healthy tissues.
- "Living Drug": Unlike a chemical drug that is metabolised and eliminated, CAR T-cells are a "living drug." They can persist in the body for months or even years, providing long-term surveillance against the cancer, which may lead to lasting remissions.
Consult a Haemato Oncologist for the best advice
How Does CAR T-Cell Therapy Work? The Step-by-Step Process
The journey of CAR T-cell therapy is complex and typically takes a few weeks. Understanding the CAR T-cell therapy process timeline can help patients and families prepare.
Step 1: Collection (Leukapheresis)
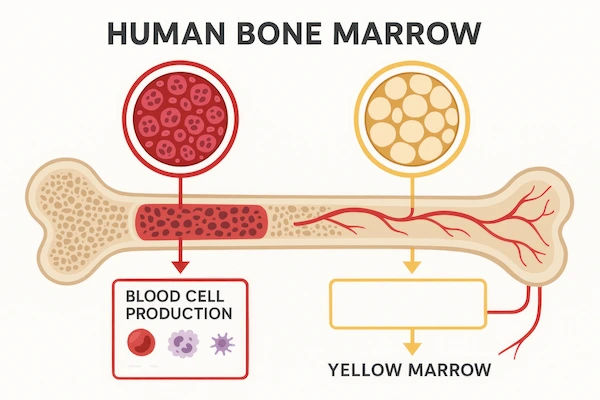
The first step is to collect the patient’s T-cells. This is done through a procedure called leukapheresis. Blood is drawn from the patient, passed through a machine that separates out the white blood cells (including T-cells), and the remaining blood components are returned to the patient. It’s similar to donating platelets and is generally not painful.
Step 2: Engineering and Multiplication
The collected T-cells are frozen and sent to a central, specialised laboratory. Here, scientists use a disarmed virus (often a lentivirus) to insert the genetic instructions for the chimeric antigen receptor (CAR) into the T-cells. These newly engineered CAR T-cells are then grown in large numbers, millions or even billions, until there is a sufficient dose for treatment.
Step 3: Conditioning Chemotherapy
A few days before the infusion of the CAR T-cells, the patient usually undergoes a short course of low-dose chemotherapy. This "lymphodepleting" chemotherapy helps create space and a favorable environment in the immune system for the new CAR T-cells to expand and work effectively.
Step 4: Infusion of the “Supercharged” Cells
The engineered CAR T-cells are thawed and infused back into the patient’s bloodstream through an IV, similar to a blood transfusion. This step itself is usually straightforward and doesn't cause immediate discomfort. The real work begins after the infusion, as the cells begin to multiply and attack the cancer.
What Cancers Can Be Treated with CAR T-Cell Therapy?
Currently, CAR T-cell therapy is primarily approved for certain types of blood cancers, also known as hematologic malignancies. These cancers often have specific, well-defined antigens that the CARs can target.
FDA-Approved Treatments
As of now, the U.S. Food and Drug Administration (FDA) has approved several CAR T-cell therapies for:
- B-cell Acute Lymphoblastic Leukemia (ALL): In children and young adults up to age 25 who have relapsed or not
responded to prior treatment. - Multiple Myeloma: For adults who have already tried several other types of treatment.
- Non-Hodgkin Lymphoma (e.g., Diffuse Large B-Cell Lymphoma): For adults whose cancer has returned or hasn't
responded to other therapies. - Follicular Lymphoma and Mantle Cell Lymphoma.
The success rate for CAR T-cell therapy in these specific cancers has been remarkable, often achieving complete remission where other treatments have failed.
Cancers Under Clinical Investigation
Research is expanding rapidly. Scientists are actively investigating CAR T-cell therapy for solid tumours, such as brain tumours (glioblastoma), pancreatic cancer, and lung cancer. This is more challenging because solid tumours have a more complex environment that can suppress immune cells, and finding a target antigen unique to the cancer (and not on healthy tissues) is difficult.
Understanding the Potential: Success Rates and Effectiveness
The effectiveness of CAR T-cell therapy has been a beacon of hope in oncology. In pivotal clinical trials for aggressive B-cell lymphomas, CAR T-cell therapy led to complete remission in 40% to 50% of patients who had no other options. For pediatric ALL, initial remission rates have been as high as 80-90%. Perhaps even more impressive is the durability of these responses; a significant proportion of these patients remain in remission years later, suggesting a potential cure for some. However, it's important to note that not everyone responds, and some may relapse. If you or a loved one are considering this path, it is crucial to consult with an oncologist specialised in immunotherapy to understand the potential outcomes for your specific situation. You can book a consultation with an expert oncologist online through Apollo24|7 to discuss your eligibility.
The Challenges: Common Side Effects and Risks
While powerful, CAR T-cell therapy can cause significant side effects, which require careful management in a specialised medical centre.
Cytokine Release Syndrome (CRS)
This is the most common side effect. As the CAR T-cells multiply and kill cancer cells, they release a flood of inflammatory proteins called cytokines into the bloodstream. This can cause high fever, dangerously low blood pressure, difficulty breathing, and organ dysfunction. While severe CRS can be life-threatening, doctors have become adept at managing it with drugs like tocilizumab (Actemra®) that block the inflammatory response.
Neurological Problems (ICANS)
Some patients experience immune effector cell-associated neurotoxicity syndrome (ICANS). Symptoms can include confusion, difficulty speaking, tremors, seizures, or even coma. The exact cause is not fully understood, but these symptoms are usually temporary and manageable with steroid treatments.
Other Potential Side Effects
Other risks include a weakened immune system and increased susceptibility to infections, low blood cell counts (cytopenias), and in rare cases, the CAR T-cells may attack healthy B-cells (which also carry the target antigen), requiring lifelong antibody replacement therapy.
Who is a Candidate for CAR T-Cell Therapy?
Currently, CAR T-cell therapy is typically reserved for patients with specific relapsed or refractory (treatment-resistant) blood cancers. Ideal candidates are those who:
- Have a cancer type with an FDA-approved CAR T-cell therapy.
- Have adequate organ function (heart, liver, kidneys) to withstand potential side effects.
- Have a good performance status (are mobile and able to perform daily activities).
- Have a strong support system, as the treatment and recovery period can be demanding.
Eligibility is determined by a multidisciplinary team at a certified treatment centre. If your condition is complex and you have exhausted standard treatments, seeking a second opinion from a specialist at a major cancer centre, accessible via Apollo24|7, can provide clarity on your options.
The Future of CAR T-Cell Therapy
The field of CAR T-cell therapy is evolving at a breathtaking pace. Researchers are working to make it safer, more effective, and applicable to more cancers.
Solid Tumours: The Next Frontier
The major challenge is adapting this technology for solid tumours. Scientists are developing "smarter" CAR T-cells that can overcome the immunosuppressive environment of solid tumours and target a wider range of cancer-specific antigens.
“Off-the-Shelf” CAR T-Cells
Currently, the therapy is personalised, which is time-consuming and expensive. The future lies in "allogeneic" or "off-
the-shelf" CAR T-cells, which are made from healthy donors and can be stored ready for immediate use, making the treatment more accessible and scalable.
Conclusion
CAR T-cell therapy represents a monumental leap forward in our fight against cancer. It embodies the shift from non-specific treatments to highly targeted, intelligent therapies that work with the body's own defenses. For many patients who had run out of options, it has offered a chance at remission and a renewed hope for the future. While challenges remain, particularly regarding side effects, cost, and expanding its use to more cancer types, the progress is undeniable. The story of CAR T-cell therapy is still being written, with each clinical trial bringing new insights and refinements. If you are navigating a cancer diagnosis, staying informed about these advancements is empowering. We encourage you to use this information as a starting point for a deeper conversation with your healthcare team about the best path forward for your individual journey.
Consult a Haemato Oncologist for the best advice
Consult a Haemato Oncologist for the best advice

Dr.sanchayan Mandal
Medical Oncologist
17 Years • MBBS, DrNB( MEDICAL ONCOLOGY), DNB (RADIOTHERAPY),ECMO. PDCR. ASCO
Kolkata
Dr. Sanchayan Mandal Oncology Clinic, Kolkata

Dr. Sanchayan Mandal
Medical Oncologist
17 Years • MBBS, DrNB( MEDICAL ONCOLOGY), DNB (RADIOTHERAPY),ECMO. PDCR. ASCO
Kolkata
MCR SUPER SPECIALITY POLY CLINIC & PATHOLOGY, Kolkata

Dr Minakshi Bansal
Paediatric Haematologist
8 Years • MBBS, MD PEDIATRICS, FIAP (PHO)
Delhi
Apollo Hospitals Indraprastha, Delhi
(25+ Patients)

Dr. Shishir Seth
Haemato Oncologist
20 Years • MBBS, MD, DM (Clinical Hematology)
Delhi
Apollo Hospitals Indraprastha, Delhi
(75+ Patients)
Dr. Satya Prasad
Haemato Oncologist
5 Years • MBBS,DNB(General Medicine) ,DM(Clinical Hematology/Hemato-Oncology)
Chennai
Apollo First Med Hospitals P H Road, Chennai
Consult a Haemato Oncologist for the best advice

Dr.sanchayan Mandal
Medical Oncologist
17 Years • MBBS, DrNB( MEDICAL ONCOLOGY), DNB (RADIOTHERAPY),ECMO. PDCR. ASCO
Kolkata
Dr. Sanchayan Mandal Oncology Clinic, Kolkata

Dr. Sanchayan Mandal
Medical Oncologist
17 Years • MBBS, DrNB( MEDICAL ONCOLOGY), DNB (RADIOTHERAPY),ECMO. PDCR. ASCO
Kolkata
MCR SUPER SPECIALITY POLY CLINIC & PATHOLOGY, Kolkata

Dr Minakshi Bansal
Paediatric Haematologist
8 Years • MBBS, MD PEDIATRICS, FIAP (PHO)
Delhi
Apollo Hospitals Indraprastha, Delhi
(25+ Patients)

Dr. Shishir Seth
Haemato Oncologist
20 Years • MBBS, MD, DM (Clinical Hematology)
Delhi
Apollo Hospitals Indraprastha, Delhi
(75+ Patients)
Dr. Satya Prasad
Haemato Oncologist
5 Years • MBBS,DNB(General Medicine) ,DM(Clinical Hematology/Hemato-Oncology)
Chennai
Apollo First Med Hospitals P H Road, Chennai
More articles from Cancer
Frequently Asked Questions
1. Is CAR T-cell therapy a cure for cancer?
While it has led to long-term remissions and is considered a potential cure for some patients with specific blood cancers, it is not universally a cure. Responses vary, and some patients may relapse. It is best described as a highly effective treatment that can induce durable remissions.
2. What is the cost of CAR T-cell therapy, and is it covered by insurance?
CAR T-cell therapy is very expensive, often costing hundreds of thousands of dollars. However, Medicare and many private insurance companies do provide coverage for FDA-approved CAR T-cell therapies for specific indications, as they are considered standard of care in those settings. Patients should work closely with their treatment centre's financial counselors.
3. How long does the entire CAR T-cell therapy process take?
From the initial T-cell collection to the final infusion, the process typically takes about 3 to 5 weeks. This includes time for cell manufacturing, which is the most variable step. Patients should also plan for a hospital stay of 1-2 weeks after infusion for monitoring.
4. What is life like after CAR T-cell therapy?
Recovery is a gradual process. Patients need close follow-up for several months to monitor for side effects and the cancer's response. Due to the effects on the immune system, precautions against infection are critical. Many patients who achieve remission can eventually return to a good quality of life.
5. Are the side effects of CAR T-cell therapy permanent?
Most side effects, including neurological symptoms, are temporary and resolve with appropriate management. However, some effects, like prolonged low blood cell counts or the need for immunoglobulin replacement due to B-cell depletion, can be long-term.